About NGGT001
NGGT001 is an investigational gene therapy drug for Bietti’s Crystalline Dystrophy, currently undergoing a Phase I/II clinical trial. The drug has successfully completed a proof-of-concept Investigator-Initiated Trial (IIT) in humans.
Clinical Trial Progress:
All patients in the IIT reported improvements in their quality of life, with their Best Corrected Visual Acuity (BCVA) increasing by an average of 14.7 letters on the ETDRS chart, while subgroup patient had an 18.9 letter increase in the BCVA. The ongoing Phase I/II clinical trial is scheduled to be completed by the end of 2024.
About Bietti’s Crystalline Dystrophy
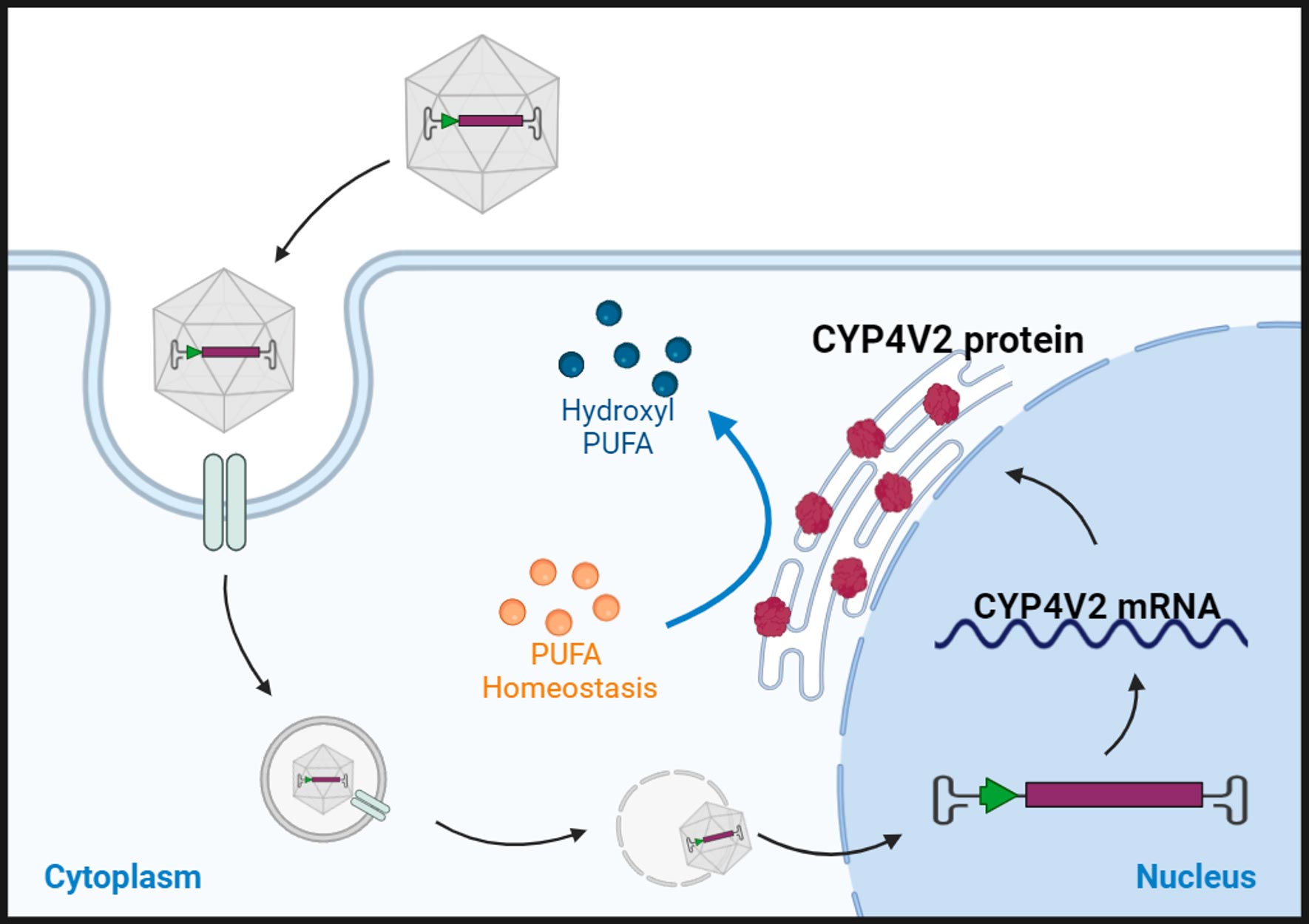
Bietti’s Crystalline Dystrophy (BCD) is a rare autosomal recessive ocular disease caused by pathogenic variants in the CYP4V2 gene and characterized by yellow-white crystalline lipid deposits in the retina and sometimes the cornea, degeneration of the retinal pigment epithelium (RPE), and sclerosis of the choroidal vessels. However, in some early phases and all late stages of BCD, the yellow-white crystalline deposit may be absent, leading to significant underdiagnosis of the disease. Carrier testing is recommended for at-risk relatives¹.
Patients can suffer from night blindness and have trouble seeing in low-light conditions. Patients may experience a blind spot near the center of the visual field, or paracentral scotoma, impaired color vision with colors appearing distorted, or reduced visual acuity and progressive peripheral vision loss. Most patients will eventually reach a stage of legal blindness as the disease progresses.
Currently, there is no approved treatment for BCD, but NGGT001 is a promising gene therapy drug for BCD which is being evaluated in an ongoing clinical trial.
About NGGT002
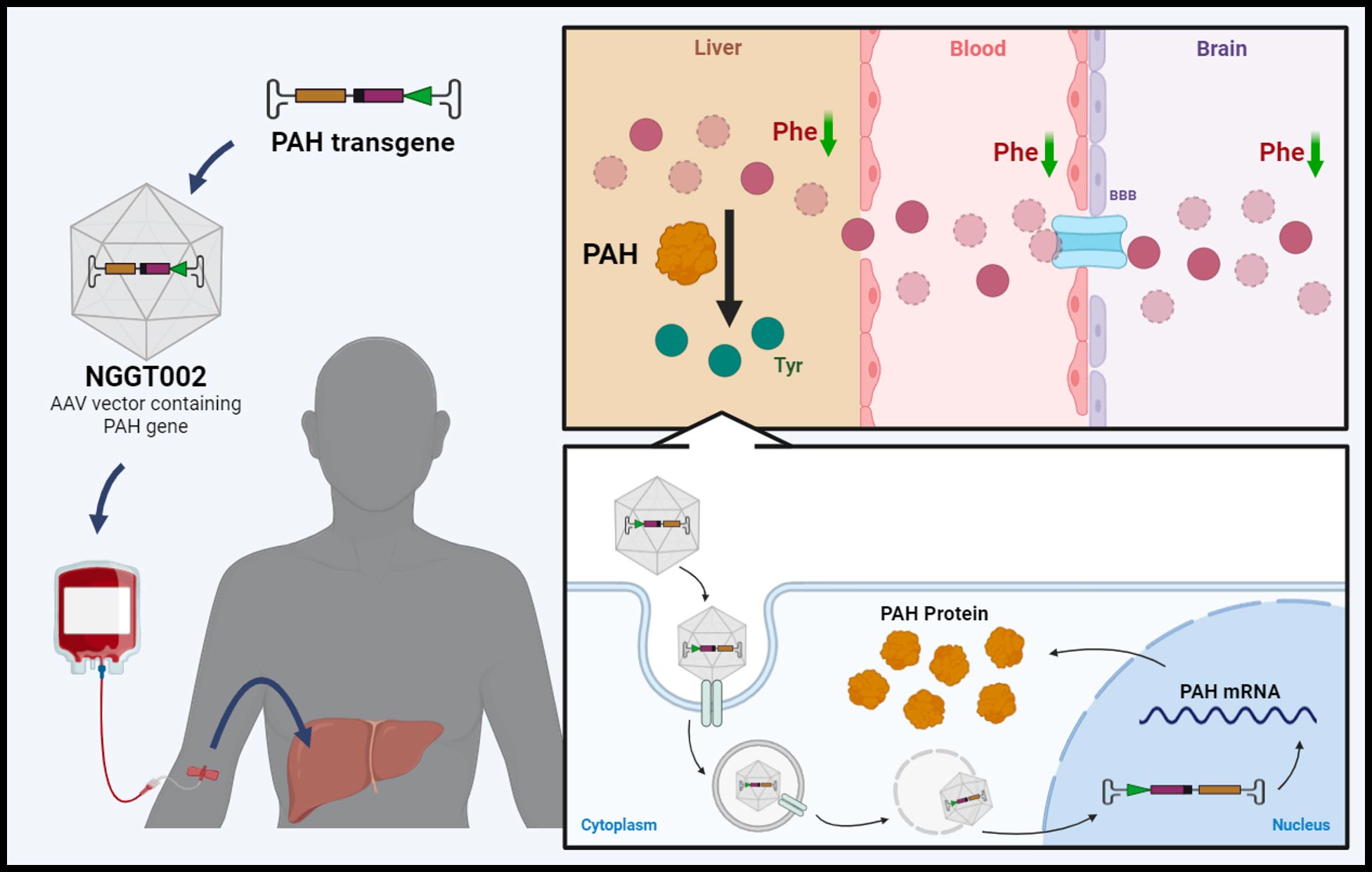
NGGT002 is an investigational gene therapy drug for Phenylketonuria, currently undergoing a Phase I/II clinical trial. The drug has successfully completed a proof-of-concept Investigator-Initiated Trial (IIT) in humans.
Clinical Trial Progress:
Four doses of NGGT002 have been tested in eight patients with classic PKU. In the high-dose cohort, five out of six patients maintained their plasma phenylalanine (Phe) around 120 µmol/L for up to 32 weeks following a single NGGT002 administration. The long-term effects of this treatment are currently being monitored and tracked. These preliminary human results will be further verified in the ongoing Phase I/II clinical trial.
About Phenylketonuria (PKU)
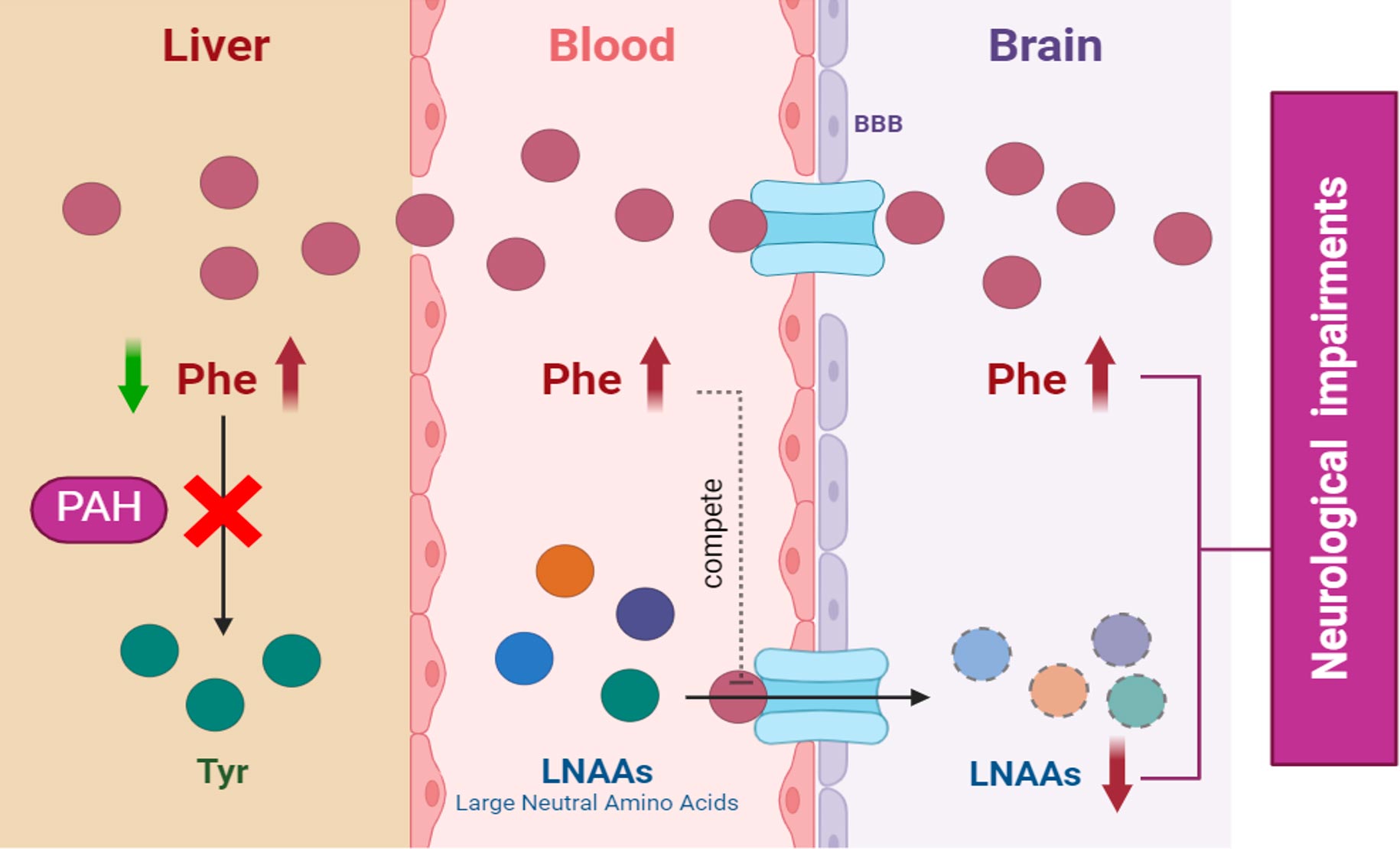
Phenylketonuria, commonly known as PKU, is a rare genetic disorder characterized by the body’s inability to break down an amino acid called phenylalanine. This condition is caused by a deficiency in the enzyme phenylalanine hydroxylase, which is necessary for converting phenylalanine into tyrosine, another amino acid. Without this enzyme, phenylalanine accumulates in the blood and brain, leading to potentially severe health problems. Ongoing research in gene therapy, enzyme replacement therapy, and other innovative treatments offers hope for improved management and potential cures for PKU. NGGT Inc. is at the forefront of this research, developing novel gene therapy solutions to address the root cause of PKU and improve the quality of life for patients.
PKU is typically diagnosed shortly after birth through newborn screening tests. If untreated, high levels of phenylalanine can cause intellectual disabilities, developmental delays, behavioral problems, and seizures. Early detection and management are crucial to prevent these complications.
The primary treatment for PKU involves a strict, lifelong diet low in phenylalanine. This diet includes specially formulated medical foods and supplements to ensure adequate nutrition while keeping phenylalanine levels under control. Some patients may also benefit from medications that help reduce phenylalanine levels.
About NGGT006
NGGT006 is an investigational gene therapy drug for familial hypercholesterolemia, which has demonstrated effectiveness in both transgenic HoFH mouse and hamster models. Three doses have been tested in four HoFH patients in a proof-of-concept Investigator-Initiated Trial (IIT) in humans.
Clinical Trial Progress:
In the high dose cohort, two of two patients experienced significant reduction in the plasma LDL-C, with levels decreasing to 3.87mmol/L after one week and 1.58mmol/L after two weeks. This represents an over 90% reduction in LDL-C, which encourages us to expedite the submission of the final Investigational New Drug (IND) application in the coming months.
About Familial Hypercholesterolemia (FH)
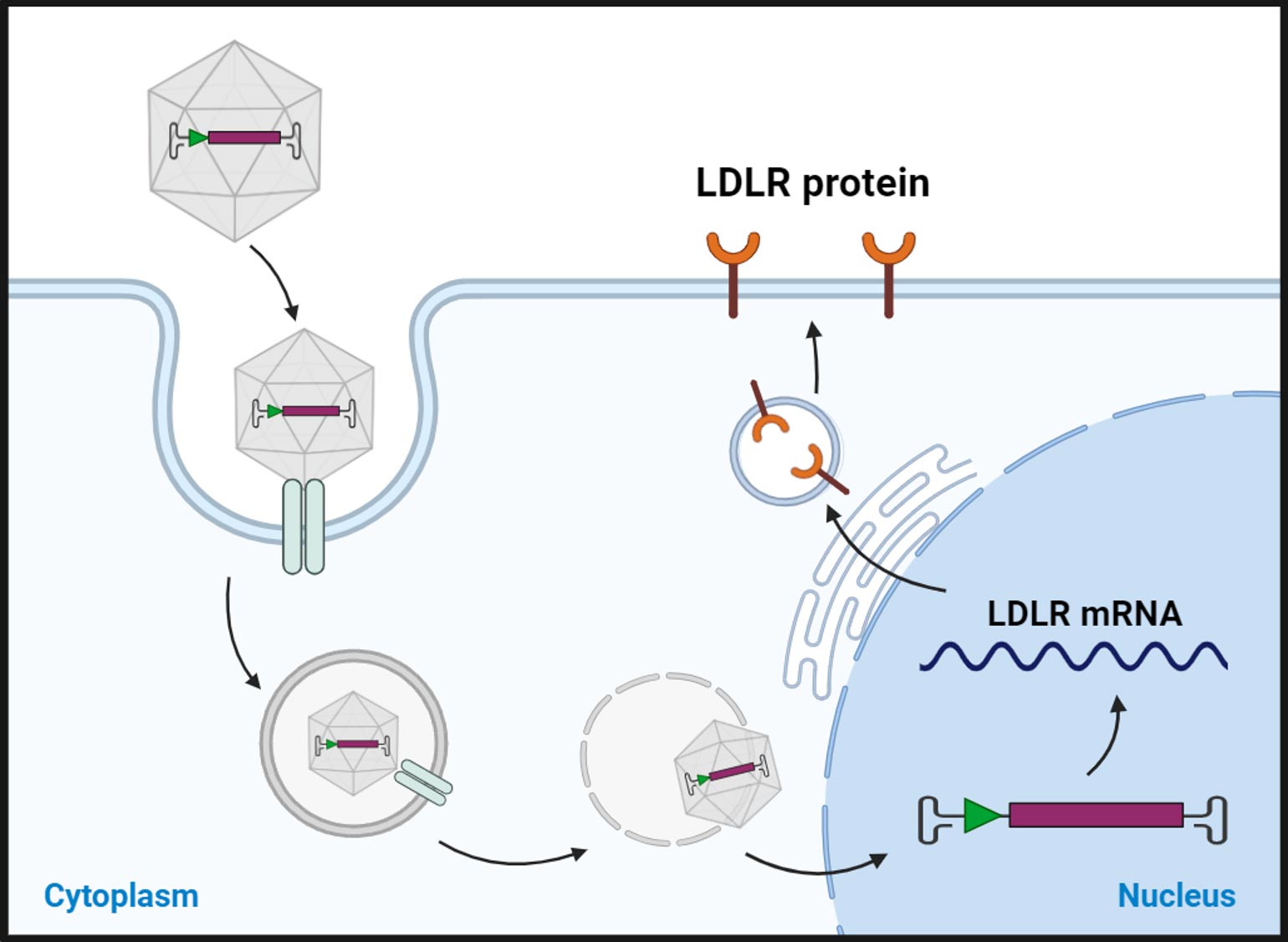
FH is a genetic disorder that leads to a significantly increased risk of heart disease, stroke, and early cardiovascular disease. FH is caused by mutations in the genes responsible for producing or regulating LDL receptors, which are responsible for removing LDL cholesterol from the bloodstream.
The estimated prevalence of Familial Hypercholesterolemia (FH) varies depending on the population studied, but it is generally estimated to affect 1 in 200-500 people worldwide, and is characterized by elevated levels of low-density lipoprotein (LDL) cholesterol in the blood. FH is a relatively common genetic disorder and has been reported in all ethnic populations. However, the exact number of people affected by FH is difficult to determine as many cases may go undiagnosed or misdiagnosed. Early diagnosis and treatment of FH is important to reduce the risk of cardiovascular disease and improve patient outcomes.
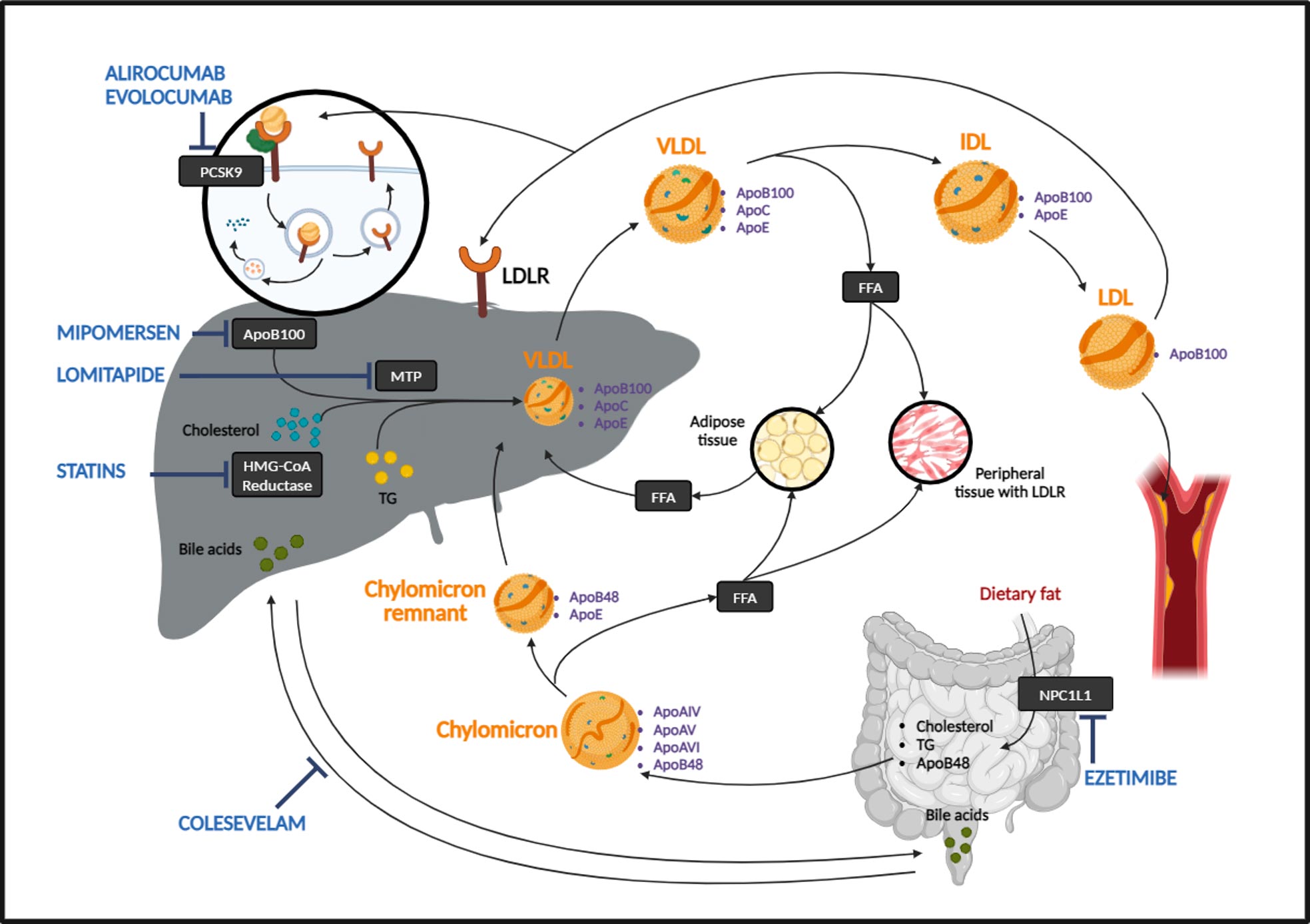
Treatment for FH includes medications to lower cholesterol levels and prevent cardiovascular disease. There are several medications used to treat Familial Hypercholesterolemia (FH). The goal of treatment for FH is to lower LDL cholesterol levels and reduce the risk of heart disease and cardiovascular disease. Some commonly used medications for FH include:
- Statins: Statins are the most commonly prescribed medication for FH. They work by blocking the enzyme in the liver that is responsible for producing cholesterol. This leads to a reduction in the levels of LDL cholesterol in the blood.
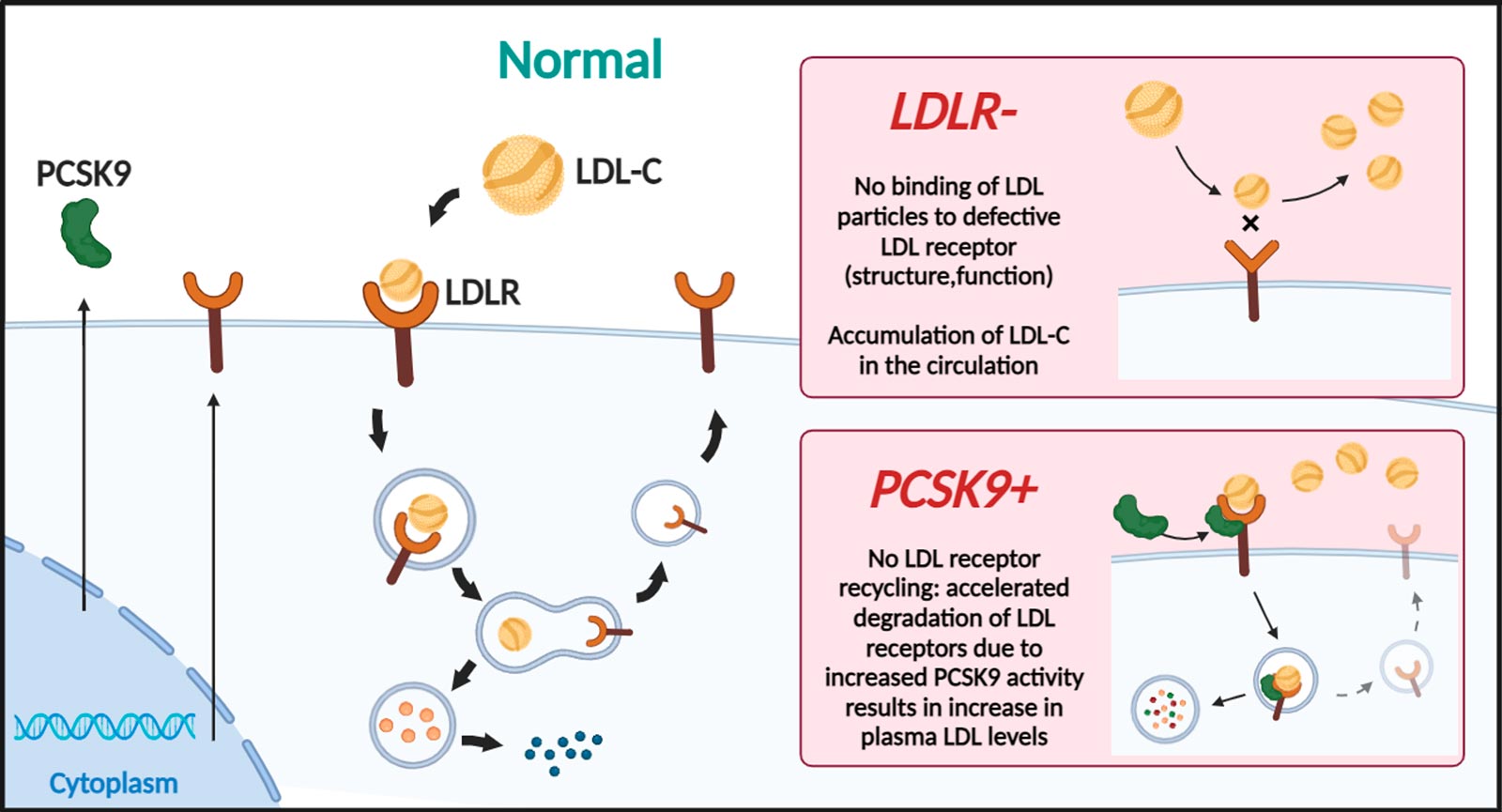
- PCSK9 inhibitors: These medications work by increasing the number of LDL receptors in the liver, allowing for more efficient removal of LDL cholesterol from the bloodstream.
- Bile acid sequestrants: These medications work by binding to bile acids in the intestine and preventing their reabsorption. This leads to an increase in the demand for cholesterol, which the liver compensates for by removing more LDL cholesterol from the bloodstream.
- Niacin: Niacin is a type of B-vitamin that has been shown to raise HDL (good) cholesterol levels and lower LDL cholesterol levels.
- Ezetimibe: This medication works by blocking the absorption of cholesterol from food in the intestine, leading to a reduction in the levels of LDL cholesterol in the blood.
These medications are typically used in combination with lifestyle changes such as a low-fat diet, regular exercise, and avoiding tobacco to achieve optimal cholesterol control and reduce the risk of cardiovascular disease in individuals with FH. The specific treatment plan for FH will depend on the individual’s age, severity of lipid abnormalities, and overall health status.
About Homozygous Familial Hypercholesterolemia (HoFH)
Homozygous Familial Hypercholesterolemia (HoFH) is a rare and severe genetic disorder characterized by extremely high levels of low-density lipoprotein cholesterol (LDL-C) from birth. It occurs when an individual inherits two defective copies of the gene responsible for regulating LDL-C levels, one from each parent. This results in significantly impaired clearance of LDL-C from the bloodstream, leading to cholesterol levels that can be several times higher than normal. Several genes have been identified to cause HoFH, namely, low-density lipoprotein receptor (LDLR 85-90%), apolipoprotein B (APOB 5-10%) proprotein convertase subtilisn/kexin-type 9 (PCSK9 1-3%)
Individuals with HoFH are at a markedly increased risk of developing early and aggressive cardiovascular diseases, including coronary artery disease, aortic stenosis, and heart attacks, often manifesting in childhood or adolescence. Without intervention, these conditions can lead to severe health complications and a significantly reduced life expectancy. Early diagnosis and aggressive treatment are crucial for managing HoFH. Therapeutic strategies typically include a combination of lifestyle modifications, high-dose statins, other lipid-lowering medications, and advanced treatments such as LDL apheresis, which is a procedure to remove LDL-C from the blood. In some cases, liver transplantation may be considered. Ongoing medical management and monitoring are essential to improve outcomes and quality of life for individuals with HoFH.
The European Atherosclerosis Society (EAS) recommended that in adult patients with HoFH (>18 year) , with the goal <1.8mmol/L, and <1.4mmol/L with additional ASCVD-risk factors (elevated Lp(a), diabetes mellitus) or established ASCVD.
However, current pharmacological treatment for HoFH can only reduce plasma LDL-C levels by approximately 50%, which is often insufficient to meet these stringent goals. As a result, achieving the recommended LDL-C targets typically requires additional interventions, such as lipoprotein apheresis or liver transplantation, both of which are more effective in significantly lowering LDL-C levels.
Gene therapy may provide another one-time alternative solution.