Introduction to Gene Therapy for Rare and Chronic Disease
Gene therapy represents a groundbreaking approach in the field of medical science, offering potential cures and transformative treatments for a variety of genetic disorders. This innovative technique involves altering the genetic material within a person’s cells to treat or prevent disease. By directly targeting the root cause of genetic disorders, gene therapy promises not just symptom management but actual cures, a paradigm shift from traditional treatments.
Understanding Gene Therapy
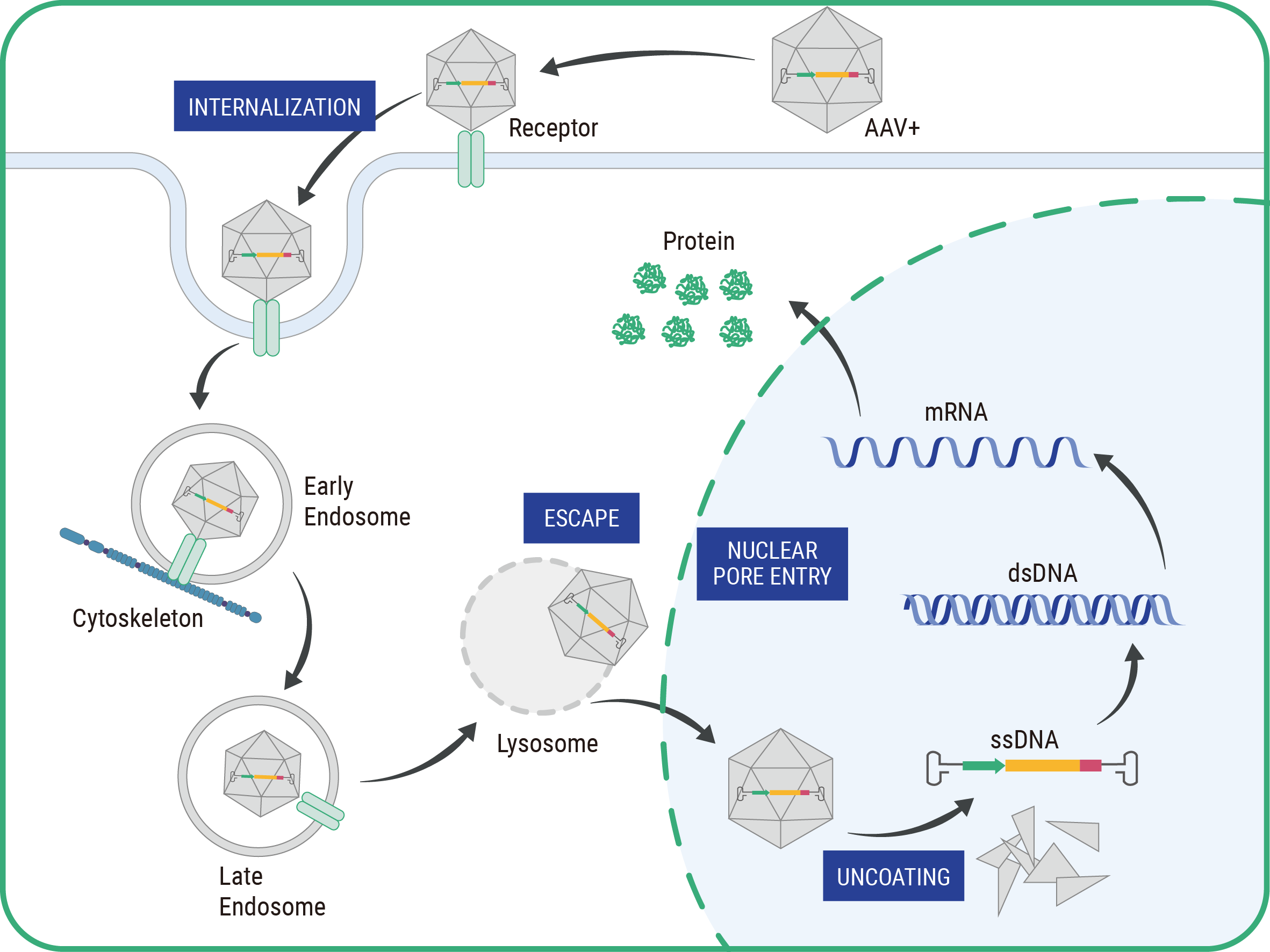
Gene therapy works by introducing, removing, or altering genetic material within a patient’s cells. The primary methods include replacing a faulty gene with a healthy copy, inactivating a malfunctioning gene, or introducing a new or modified gene into the body to help treat a disease. These techniques utilize vectors, often viruses, engineered to safely deliver the genetic material into the patient’s cells.
Impact on Rare Diseases
Rare diseases, often referred to as orphan diseases, affect approximately 400 million people globally. There are over 7,000 identified rare diseases, yet approximately 95% of them currently have no approved treatment. While rare, these conditions can lead to severe health complications and significantly reduced quality of life for patients. Traditional treatments that do exist for rare diseases are limited, frequently focusing on managing symptoms rather than addressing the underlying genetic cause. Gene therapy offers hope by targeting the genetic defects directly, potentially providing lifelong cures from a single treatment. Success stories, such as the treatment of spinal muscular atrophy (SMA) and certain inherited retinal diseases (Rep65 LCA), underscore the profound impact gene therapy can have on patients with rare genetic disorders. By addressing the root cause, gene therapy holds the promise of transforming the lives of millions who suffer from these debilitating conditions, offering a new era of hope and possibility.

Applications in Chronic Diseases
Chronic diseases, such as wet age-related macular degeneration (wet AMD), neurodegenerative diseases, heart disease, diabetes, and certain cancers, pose significant challenges due to their long-term nature and complex etiology, yet have verified pathogenetic mechanisms. While gene therapy’s role in treating chronic diseases is still emerging, its potential is immense. By addressing genetic predispositions and modifying disease pathways at the molecular level, gene therapy could revolutionize the management of chronic conditions. For example, recently developed gene editing technologies like CRISPR-Cas9 are being explored to correct genetic mutations responsible for conditions like cystic fibrosis and sickle cell anemia, offering the possibility of sustained relief and improved quality of life. Moreover, research is advancing to apply gene therapy to neurodegenerative diseases to potentially halt or reverse the progression of disorders such as Parkinson’s and Alzheimer’s. These advancements highlight the versatility of gene therapy and its capacity to not only manage but potentially cure chronic diseases, paving the way for a new era of medical treatment.
Challenges and Future Directions
Despite its promise, gene therapy faces several challenges, including successful delivery, immune responses to treatment, and ethical considerations. Ensuring long-term efficacy and safety remains a critical focus of ongoing research. As scientists and clinicians continue to refine these technologies, collaboration between regulatory bodies, researchers, and patient advocacy groups is essential to navigate these challenges and bring gene therapy to broader patient populations.


Gene therapy stands at the forefront of a new era in medicine, with the potential to change the landscape of treatment for rare and chronic diseases. Gene therapy has the potential to transform patients who rely on constant medication and frequent hospital visits into individuals who no longer need ongoing medical treatment, allowing them to lead normal, healthy lives. By addressing the genetic foundations of these conditions, gene therapy not only offers hope for cures but also opens new avenues for personalized and precise medical care. As research progresses, the continued evolution of gene therapy holds promise for countless individuals worldwide, transforming lives and redefining possibilities in healthcare.
— Lixin Jiang, CEO
Advanced cGMP Manufacturing Powerhouse
Talented and Experienced Team:
Our NGGT team comprises seasoned experts with over 30 years of experience in large-scale preparation of recombinant adeno-associated virus (rAAV), who have played critical roles in the development and commercialization of two marketed gene therapy drugs.
High-Tech Facility:
Our 90,000-square-foot, state-of-the-art cGMP facility is designed to support every stage of rAAV manufacturing. From plasmid preparation and viral vector production to final filling, our comprehensive in-house solutions ensure seamless and efficient operations.
Seamless Quality Control:
We prioritize quality and efficiency through our integrated operations, ensuring that every step of the manufacturing process meets the highest standards.
Regulatory Excellence:
In the past 18 months, two of our Investigational New Drug (IND) applications were approved by the FDA without any challenges in Chemistry, Manufacturing, and Controls (CMC). We are on track to submit two more INDs in the coming 18 months, continuing our commitment to regulatory excellence and innovation.




Our technology
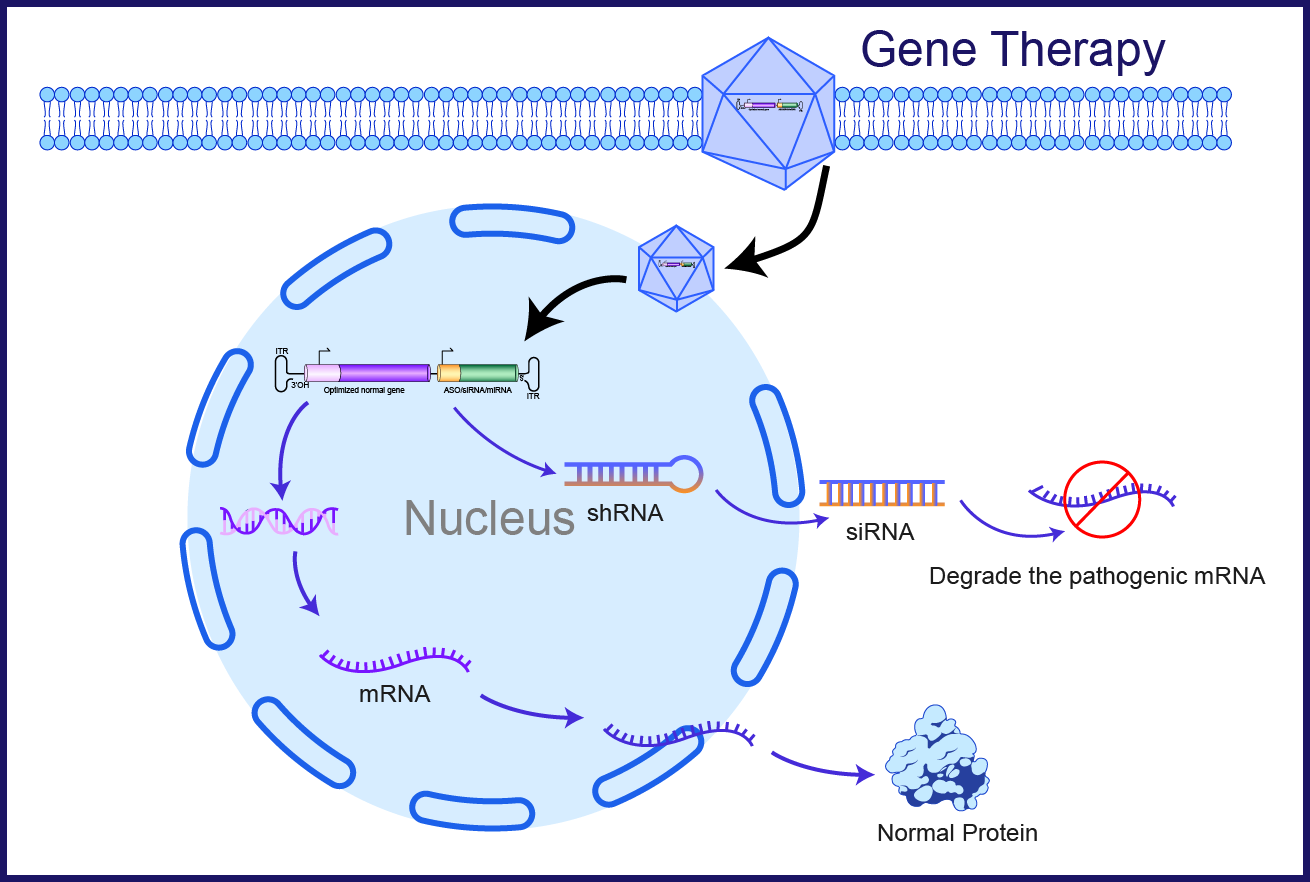
The concept “One target, two bullets” is based on a dual function vector comprised of a nucleic acid configured to express at least two active agents.
- “Bullet 1” expresses siRNA via shRNA or miRNA sequences in the vector for silencing endogenous mRNA from the targeted gene.
- “Bullet 2” expresses the correct version of the protein from the mutated gene being silenced, which is insensitive to the silencing activity of Bullet 1.
Our vector is designed to achieve an optimized therapy by reducing endogenous toxic mutants as well as WT gene expression of the targeted protein, while maintaining protein level and activity by introducing a genetically engineered copy of the targeted gene.
Our U.S. patent also describes methods of making dual function vectors and methods of using vectors for treatment of diseases and to create animal models.
Legend for the figure: This figure shows a dual function vector for the treatment of single gene hereditary diseases caused by autosomal dominant negative, X linked dominant or X-linked recessive mutations by maintaining protein level and activity by introducing a targeted gene copy.